What is the atomic number of a neutral atom with 11 protons 12 neutrons and 11 electrons. What is the number of protons in this atom.

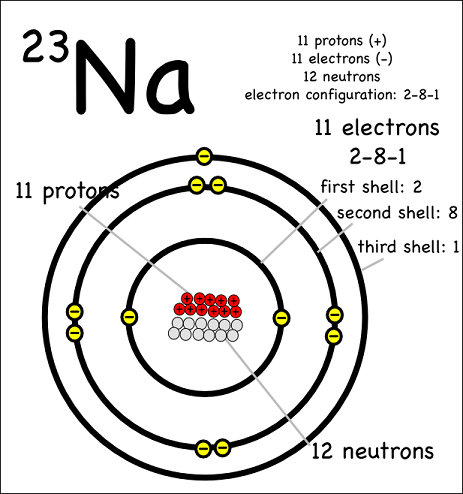
Sodium Bohr Model How To Draw Bohr Diagram For Sodium Na Atom
For example the atomic mass of chlorine Cl is 3545 amu because chlorine is composed of several isotopes some the majority with an atomic mass of 35 amu 17 protons and 18 neutrons and some with an atomic mass of 37 amu 17 protons and 20 neutrons.

. This was not asked but it completes the question. But the number of nucular protons ie. If it has 11 electrons it has 11 protons which makes it atomic number 11 and atomic symbol Na.
A neutral atom with 11 electrons would by definition have to have 11 protons. 2 How many neutrons does an atom have if its atomic number is 12 and its mass is 25. What is the number of protrons in this atom is.
How does a Sulfur-2 ion differ from a neutral Sulfur atom. Thallium has two isotopes thallium-203 and thallium-205. This tells us that sodium has 11 protons and because it is neutral it has 11 electrons.
19K views View upvotes. We know that the atomic number of sodium is 11. A neutral atom contains 11 protons 12 neutrons and 11 electrons.
What is the charge of an atom with 12 protons and 10 electrons. Click hereto get an answer to your question A neutral atom has 12 electrons and 13 neutrons. A neutral atom contains 12 neutrons and 11 electrons.
3 How many neutrons are in an atom with an atomic number of 25. It is asoft and silver-white reactive metal of the alkali metal group. The atomic mass of the atom is.
The term we would use for this element is 23N a. How may electrons in the neutral atom. The mass number of an element tells us the number of protons AND neutrons in an atom the two particles that have a measurable mass.
Atomic mass Noof protons No. What is its atomic number and its mass number. Of Neutrons 1112 23 Hence the correct option is A.
Neutrons plus protons The number of neutrons in the nucleus of an atom can be determined by Subtracting the atomic number from the mass number of the atom A neutral atom contains 12 neutrons and 11 electrons. The element with 12 neutrons and 11 electrons is sodium. A neutral atom contains 12 neutrons and 11 electrons The number of protons in from PHYSICS 1 at Western University.
An atom contains 11 protons and 12 neutrons. The atom is of- a potassium b sodium c lithium d magnesium. A neutral atom contains 12 neutrons and 11 electrons.
A 23 B 30 C 22 D 24 Medium Solution Verified by Toppr Correct option is A The atomic mass of an atom is the sum of protons and neutrons in an atom. The number of protons in this atom is 11 What is the number of electrons in a neutral atom of Fluorine 9. If it has 12 protons that means it has 12 units of positive charge and 10 electrons which means 10 units of negative chargeTherefore net charge 12-10 2 2 units of positive chargeSince it has 12 protons that means its atomic number is 12 and we know the element with atomic number 12 is.
What is the mass number of a chlorine atom that has 17 protons and 18 neutrons. Thalliums atomic number is 81 and. Z 11 for sodium.
1 What element has an atomic number of 12 and a mass number of 25. Its mass number is. Atomic Number number of protons 11 Mass number number of protons number of neutrons 11 12 23 Number of electrons number of protons so the atom is neutral.
The sum of neutrons and protons the massive nuclear particles gives the mass number with which we often label the elemental symbol as a left hand superscript. 5 What has 12 protons and electrons. If it is a 23N a nuclide there must be 12 neutrons in the nucleus.
Its mass number is. Calculate its mass number. The number of protons in this atom is 1 1 3 12 2 11 4 23 2 Isotopes of an element must have different.
If it is a sodium atom then Z 11 by definition. This tells us that sodium has 11 protons and because it is neutral it has 11 electrons. Z UNEQUIVOCALLY identifies the atom.
What is the atom. The Atom is neutral Sodium 23. A neutral atom has 1 2 electrons and 1 3 neutrons.
If there are 11 protons THERE MUST be 11 electrons orbiting around the atom because the element here is NEUTRAL. See answer 1 Best Answer. An atom has 19 protons and 20 neutrons.
In this case. 1A neutral atom contains 12 neutrons and 11 electrons. It would be worth your while to review these definitions.
We know that the atomic number of sodium is 11. 4 How many neutrons are in the nucleus of an atom that has a mass of 36 and an atomic number of 25. Ratio of neutrons to protons 4 ratio of electrons to protons Note that questions 30 and 31 have only three choices.
An atom of an element has 11 protons 11 electrons and 12 neutrons. Join Login 11th Chemistry Structure of Atom Atomic Number and Mass Number A neutral atom has 12 elect. Sodium has an atomic weight of 229898 which means if it has 11 protons it would have 12 neutrons to equal that weight.

A Neutral Atom Contains 12 Neutrons And 11 Electrons The Number Of Protons In This Atom Is 1 1 3 Brainly Com

Atomic Structure Chemistry Ppt Download
An Atom Contains 11 Protons 11 Electrons And 12 Neutrons What Is Its Atomic Number And Its Mass Number Quora
0 Comments